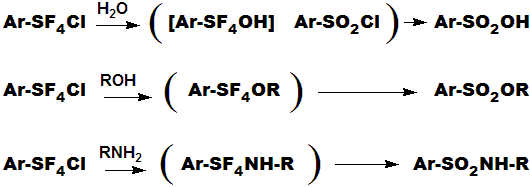Aromatic SF5 Perfluorosulfanate Pentafluorosulfanate Sulfanyl
Pentafluoride Sulfurpentafluoride Perfluorosulfanyl
Pentafluorosulfanyl SF5 Lloyd Garrick explosives explosive
propellant rocket fuel high energy FLUOLEAD agricultural
pharmaceutical medicinal drug drugs
Novel, New Aromatic SF5 Derivatives ! |
Prepared in High Yield via Highly Versatile & Cost Competitive Methods |
Aromatic SF5
compounds are expected to be useful as BUILDING BLOCKS for
pharmaceutical, agricultural and other bio-active agents, pesticides,
liquid crystals, novel structural and conductive polymers,
dyes, organic semiconductors, high energy compounds, propellants and
explosives, and many other higher performance organic materials.
Many SF5 containing intermediates have recently become available, but only in small quantities priced at hundreds to thousands
of dollars per gram, currently made by inefficient and expensive processes. The new recently developed procedures
(described below) when commercialized, promise to make these compounds more readily available, much more affordable, and in
greater variety for research and production of many useful products.
A new era in chemistry has begun, with the introduction of the first new functional group in over a century!
I {Lloyd Garrick} prepared the compounds below [as well as many others not listed]
in 10-100 g. quantities as part of my Research and Process Development
work at UBE America Inc., in Denver. The facility has been closed down
as of August 2013. Research,
Development and Production of these type compounds is no longer in
progress in this country to any significant extent, as far as I know at
this time.
The compounds were made either directly by the new process(es) from the
appropriate thiol or disulfide starting material, or, in many cases, by
standard derivatizations of ring-fluorine containing compounds. Other
common manipulations (nitration, metalation, methyl oxidation),
were also used; SF5 is inert to all but the most drastic reaction conditions.
In addition, I have a number of new research ideas which, if successful, would be improvements even on these "new" procedures,
and would also skirt the patents. They haven't been tested yet; I need a lab!
Sooner or later some forward-thinking non-risk-averse entrepreneurial company is going to give me a lab to develop this chemistry, and they are gonna get the goods!
Everyone else left in the dust!
My new (unpublished, unposted) procedure(s) can make for you the world's most powerful non-nuclear explosive:
 as well as some seriously impressive bioactives (pharmaceuticals, agricultural, etc.). So is NY1 interested or what !?
as well as some seriously impressive bioactives (pharmaceuticals, agricultural, etc.). So is NY1 interested or what !?
Hey China (India? Any others??) - you gotta be working on this now! I can't come over there, but if you or a
client company can set me up in a facility here I'll give it all to you! Hell, I'm not proud - nothing seems to
happening in this country!
|
 |
| LLOYD GARRICK |
 |
| |
Introduction:
Concurrent with significant developments in the synthetic methodology for the preparation of SF5 containing compounds, many potential applications, derived from the interesting and unique
properties of the SF5 function, have been proposed,
particularly in certain advanced specialty chemical fields such as
pharmaceuticals, agrochemicals and electronics.
|
| |
The SF5 function, one of the most electron-withdrawing groups known, imparts outstanding lipophilic properties
to compounds which
incorporate it, as well as added chemical and thermal stability. It is
expected that the higher lipophilicity and other properties of SF5
compounds will show interesting and unique influences on biological activities other than those observed with fluorine or trifluoromethyl-groups. |
 |
| LLOYD GARRICK |
Regarding electronics chemicals, it is reported that there has been a rapid increase in the number of patents which list
the SF5 group in liquid crystals due to the strong dipole moment which can be achieved by the SF5 group. |
Properties of Aromatic SF5 compounds: |
| SF5 group is called "Super-trifluoromethyl group", and the expected properties of SF5-containing
compounds are similar to
the ones which are seen in general fluorine compounds, although most of
them are significantly enhanced by the increment of the number of
fluorine atoms in SF5 group. |
| |
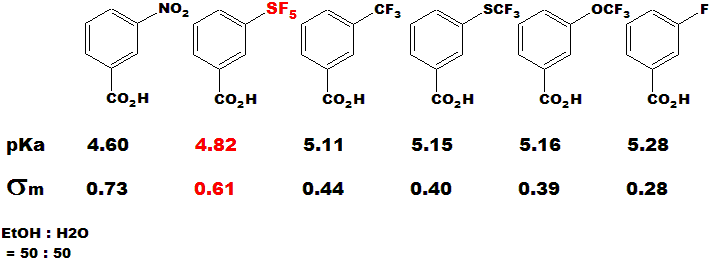
Electron-withdrawing Effect
SF5 group is recognized as a strong electron-withdrawing
group. Figure below shows the comparative values of pKa in the substituted benzoic acid derivatives which have SF5, CF3, SCF3, OCF3 and
F, respectively. The SF5 derivative is ranked as the second strongest group after the nitro-substituted one. |
 |
| LLOYD GARRICK |
Lipophilicity
It is well known that compounds which incorporate fluorine(s) show greater lipophilicity. SF5 substituted compounds are expected to show excellent lipophilicity
compared with other fluorine-containing compounds. The lipophilicity indices of several functional groups are shown below.
These values are calculated by formula from the Xow-values (octanol/water partition coefficient).
In particular in agrochemistry [agricultural chemistry, compounds such
as herbicides, fungicides, insecticides, plant growth regulators, etc.]
this quality of lipophilicity is often exploited, as it is very
important. Many examples of agents bearing CF3, OCF3 and SCF3 groups
are in use or still being developed. Illustrative examples are displayed elsewhere on this page.
|
| |
 |
| LLOYD GARRICK |
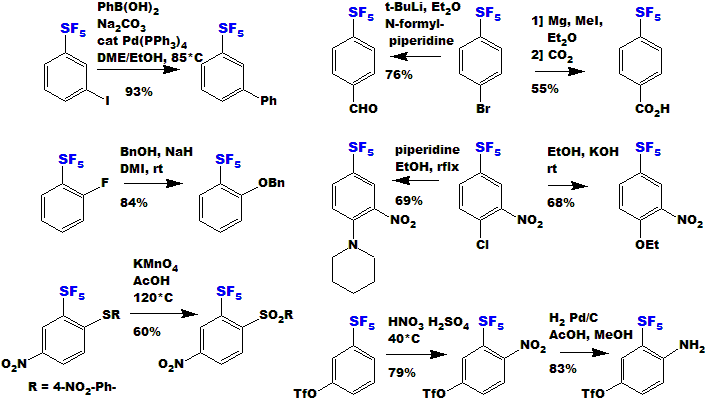
Thermal and Chemical Stability
Aromatic SF5 compounds possess excellent thermal and chemical stability.
For example, it was demonstrated that the thermal decomposition rate of PhSF5 (PSF) was less than 20% after it was heated
in a sealed tube at 400*C for 7 hours. It was also demonstrated that aromatic SF5 compounds are more tolerant than aromatic CF3 compounds
under strong conditions of Bronsted acids and bases, and can be widely applied for common synthetic transformations in high yield.
Examples of reactions for Aromatic SF5 compounds are shown below.
|
| |
 |
| LLOYD GARRICK |
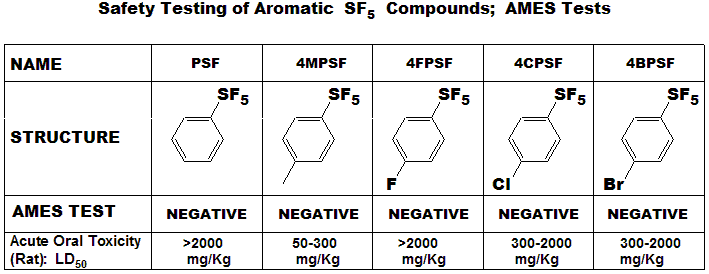
Toxicity
Regarding the assessment of toxicity of Aromatic SF5 compounds, the aromatic SF5
compounds shown below were assayed for both
Ames and Acute Oral Toxicity. Table below shows both results including
the empirical data obtained from the Acute Oral Toxicity test. 4MPSF
showed weak toxicity, with range 50-300mg/Kg and ranked as Category 3 in
UN GHS. This, and other data so far, have shown that SF5 has little (if any) intrinsic
toxicity, and it isn't metabolized.
Thus it should be a significant improvement in bioactive compounds, where it can replace toxic groups like NO2, Br and CN. |
| |
 |
| LLOYD GARRICK |
ALL of the Chloropentafluorosulfanate compounds [Ar-SF4Cl], even the crystalline solids, are extremely noxious ! They have strong, penetrating,
persistant, irritating mustard like odors. If/When you succeed in making any, you will know it ! Skin contact will produce irritation and blistering.
They are not "alkylating agents" in the classical sense, but rather
"sulfonating agents" as depicted in the equations just below.
Electron withdrawing groups on the ring tend to make the compounds more stable and less reactive; electron donating groups have the opposite
effect of course.
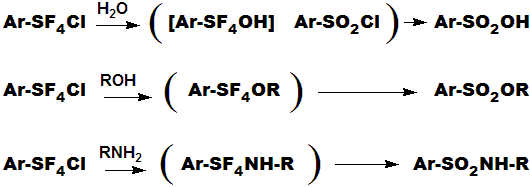
In contrast, the Pentafluorosulfanate [SF5] compounds are quite pleasant. The inert SF5 substituent gives it's molecules crystallinity,
volatility, stability, and a host of other pleasant attributes, including odor. PhSF5 is a dense (1.55) liquid with an odor between toluene
and lemon juice. Many of the solids and polyfunctional SF5 compounds resemble camphor, mint, clove, etc.
|
| LLOYD GARRICK |
| |
Hydrolytic Stability and Physical Characteristics
- ArSF5 can be held for 4 hours at 100*C in 1N NaOH without measurable hydrolysis, whereas ArCF3 is well known to be succeptible to alkaline hydrolysis, especially if ortho or para to a ring hydroxyl.
- In a 2.0 N NaOH solution at room temperature, 4-CF3-aniline readily hydrolyzed whereas 4-SF5-aniline was recovered in high yield (91%).
- ArCF3 in conc. H2SO4 at 90*C is completely hydrolyzed within minutes. Under similar conditions [such as my nitration reactions on PhSF5], no significant hydrolysis occured; mono-nitration product(s) always recovered in ~quantitative yield.
- Although SF5 appears to be stable to even strong alkaline condiditons, very vigorous acid conditions will hydrolyze it; for example, ArSF5 in 90% HNO3 / 30% Oleum at 80*C for 4-7 days [my reaction for di-nitration of PhSF5] gave about 60% hydrolysis to the sulfonate, and product(s) recovered were about 40%.
- The volume of SF5 is larger than CF3 and slightly smaller than tert-butyl:
Lentz&Seppelt, In Chemistry of Hypervalent Compounds, K. Akiba(Ed.), Wiley-VCH, New York, 1998, 295
- The SF5 group is more electronegative than CF3:
Sæthre, Berrah, Bozek, Børve, Carroll, Kukk, Gard, Winter&Thomas, J.Am. Chem. Soc. 2001,123, 10729;
Wipf, Henninger&Geib, J. Org. Chem. 1998, 63, 6088
- Unlike CF3, the SF5 group is very stable under strong acid and basic conditions:
Bowden, Comina, Greenhall, Kariuki, Loveday&Philp, Tetrahedron 2000, 56, 3399
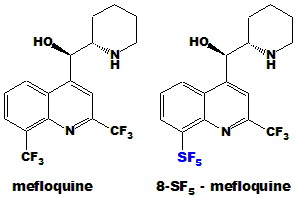
- The utility of SF5 derivatives in drug discovery was recently showcased by Wipf and coworkers in the design of improved Mefloquine:
Wipf, Mo, Geib, Caridha, Dow, Gerena, Roncal&Milner, Org. Biomol. Chem. 2009, 7, 4163
|
| |
Biological Activities
Organopentafluorosulfanyl Chemistry
The pentafluorosulfanyl (SF5) group is one of only a very few truly new functional groups to be
introduced to the armentarium of the synthetic organic chemist in the last 100 years. The pseudooctahedral symmetry of the SF5
group, presenting a square pyramid of electron density, as defined by
the fluorine ligands, is not otherwise known to the medicinal or
pharmaceutical chemist. However only with the recent availability of
the necessary reagents and building blocks has this functional group
found applications as an aromatic substituent in agrochemicals,
pharmaceuticals and liquid crystals. In aliphatic chemistry,
pentafluorosulfanylated materials are even more rarely encountered with
applications largely limited to polymer or oligomer preparations. The
SF5 group is profoundly electron withdrawing but with the highly polarizable carbon-sulfur bond may directly influence reactivity
in a manner different from that associated with the trifluoromethyl group.
Treflan Analogs
The synthesis of 2,6-dinitro-4-pentafluorosulfanyl-N,N-dipropylaniline, 2,
was achieved in a straightforward manner from commercially available
1-nitro-4-pentafluorosulfanylbenzene. In post-emergence screening 2
was found to be approximately twice as potent as trifluralin with the
same general spectrum of activity. In contrast, in pre-emergence tests,
2 was nearly 5 fold more potent against quackgrass and crabgrass. References
Serotonin Analogs
The trifluoromethyl group of
fluoxetine and fenfluramine and norfenfluramine was substituted by the
pentafluorosulfanyl group. On examination of the efficacy of the
pentafluorosulfanyl containing compounds as inhibitors of
5-hydroxytryptamine receptors, it was found that substitution could lead
to enhanced selectivity and in the case of the pentafluorosulfanyl
analog of fenfluramine, led to significantly enhanced potency against
the 5-HT2b, 5-HT2c and 5-HT6 receptors. References
Antineoplastic Agents:
Much potential for SF5 exists in the field of chemotherapy, and it is largely unresearched (ie., wide open) right now. It is well known that the internal environments
of neoplasms (tumor cells etc.) are particularly lipophilic; thus the SF5
function, as it confers strong lipophilic character to molecules
incorporating it, should offer considerable improvement in these
therapeutics, particularly those that target neoplasms in lipophilic
tissues and environments, such as brain
and CNS. References
Cannabinoid Receptor Ligands:
An array of cannabinoid ligands, bearing meta- and para-substituted pentafluorosulfanyl (SF5) aniline groups in position 3 of the
pyrazole ring, was efficiently synthesised and compared with the exact trifluoromethyl and tert-butyl analogues.
In general, the SF5 substituted ligands showed higher lipophilicity (i.e. log P values) than the CF3 counterparts and lower lipophilicity
than the tert-butyl ones. In terms of pharmacological activity, SF5 pyrazoles generally showed slightly higher or equivalent
CB1 receptor affinity (Ki), always in the nanomolar range, and selectivity towards the CB2 relative to both CF3 and tert-butyl
analogues. Functional ß-arrestin recruitment assays were used to determine equilibrium dissociation constants (Kb) and
showed that all of the tested SF5 and CF3 compounds are CB1 neutral antagonists.
These results confirm the possibility of successfully using an aromatic SF5 group as a stable, synthetically accessible and effective
bioisosteric analogue of the electron-withdrawing CF3 group, and possibly also of bulky aliphatic groups, for drug discovery and development applications.
References
Agricultural Compounds
The SF5 moiety should find considerable utility in the
agricultural (herbicide, pesticide, insecticide, fungicide etc.) sector,
perhaps more so and sooner than in pharmaceuticals,
as SF5 will confer many desired properties to these
bio-active molecules, and they do not require the lengthy (and
expensive) trials mandated with compounds for human use. References [I'll be adding more as I find them]
There are many examples of agricultural compounds now in use or being developed, which employ the CF3 function, or Cl, Br, etc., where a large electronegative lipophilic group is needed.
It is a virtually certain bet that replacement of these group(s) with SF5 would yield a much better and more potent compound.
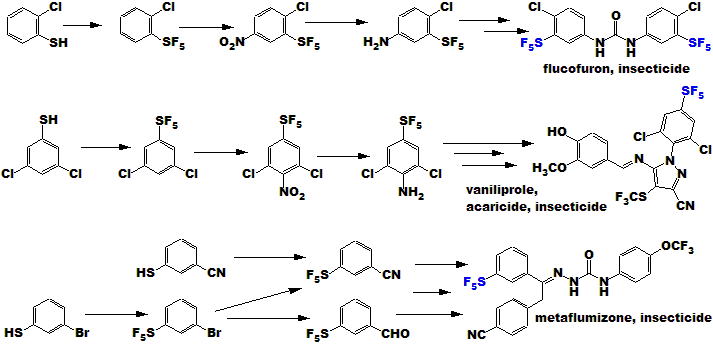
Agricultural compounds are also usually simpler than human
pharmaceuticals; the chemistry is thus more direct and simple. Some
contemporary examples are shown just below, also the
Fipronil example a little further down:
 LLOYD GARRICK LLOYD GARRICK
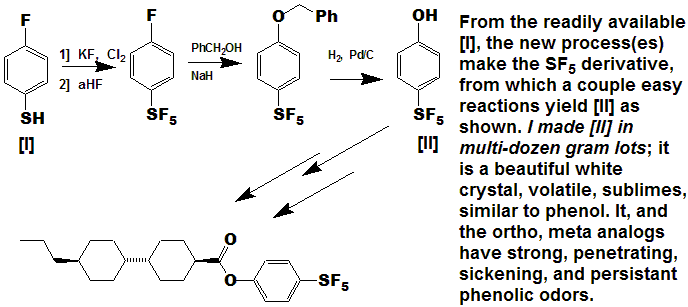
Liquid Crystals
Another wide open area of research with much growth potential; the SF5 group imparts a strong crystalline nature to compounds,
they also tend to be lower melting and more volatile than one would expect from the molecular weight; in addition, the SF5 substituent
can impart a very strong dipole moment to a molecule, critical for liquid crystals: References
 LLOYD GARRICK LLOYD GARRICK
High Tech and Specialty Polymers; other Chemistry uses
Some research has been done, much more is needed, as this area offers
much promise for thermally and chemically stable polymers; the SF5 group,
due to it's tendency to impart strength and crystallinity to it's compounds, may also have value in high strength polymers.
References
Environmental Considerations
Since SF5 does not occur in nature, it is rationally
expected that there are no enzyme systems to handle it, nor any general
bio-metabolic mechanisms to
deal with it. This, along with it's extreme chemical inertness, might
suggest that SF5 would build up in the environment and accumulate.
However, some research has already been done, and more will be in the future. What has become known at this time is that SF5 does indeed break down in the open environment, particularly
under the influence of sunlight.
The products formed are the corresponding sulfonate (ArSO3H) compounds (generally innocuous), and HF (instantly buffered to fluoride, which is ubiquitous in nature anyway). References
Currently, the introduction of fluorine into organic molecules has
become very common methodology in biomedical fields, and numerous
fluorine containing molecules have been developed and many have shown
significant promise and advantages in this field.
In particular, the pentafluorosulfanyl (SF5) group, which is a highly fluorinated functional group, has shown remarkable activity in
biochemical molecules. The introduction of the SF5 group brings not only the novel properties which originate from Fluorine element (Strong
electronegativity, high lipophilicity and high chemical stability) to the molecule, but also a larger steric effect than the CF3 group, which
is also recognized as a highly fluorinated functional group. The relative steric demand of the SF5 group is slightly less than that of a
tert-butyl group and considerably larger than that of a CF3 group. Examples of biological activities comparing the CF3 substituted agent
vs. the SF5 analog are shown below;
|
|
Mefloquine is used for both treatment and prophylaxis of malaria. 8-SF5-Mefloquine showed a longer half-life(68h) than
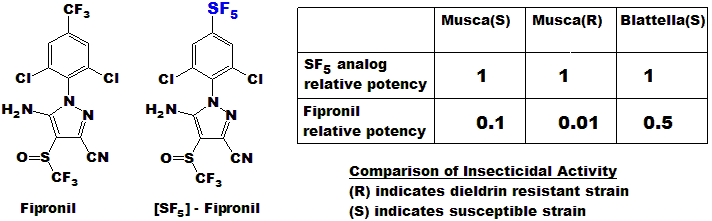
Mefloquine(23h) after administration to mice. Fipronil is a
broad spectrum insecticide. The SF5 analogue of Fipronil was not
only more active than Fipronil but showed no loss of potency towards the
resistant strain of housefly, in contrast to the Fipronil.
|
 |
| LLOYD GARRICK |
 |
| |
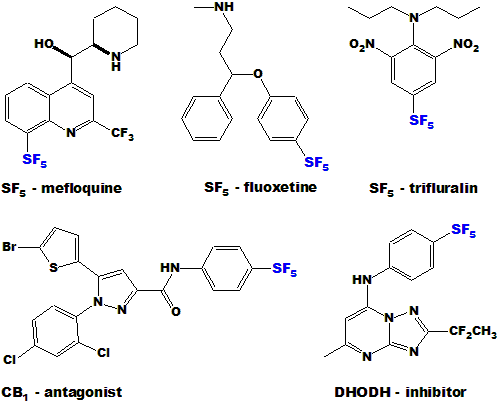
| These 5 are examples of SF5 analogs of current bio-active compounds, all of which are better than the originals:
|
 |
| LLOYD GARRICK |
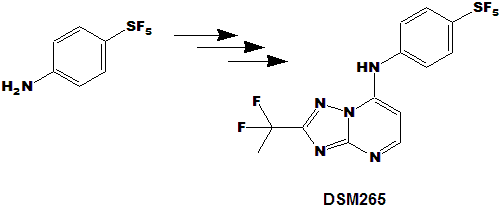
| A new anti-malarial drug (DSM265), very promising so far and currently in clinical trials, contains a p-SF5-anilino substituent as shown: |
 |
| LLOYD GARRICK |
The synthesis [see DSM265 links just below] involves replacement of chlorine on the triazolopyrimidine ring with
para-amino phenyl pentafluorosulfanate, a compound I made in 50 gr. lots (new process),
but which is now only available in gram quantities and prohibitive price (old/current procedures).
This is a perfect example of how a new and very promising drug will (would) be prohibitively expensive if it employs the SF5 function now,
but with the new SF5 processes will be much cheaper and more available! Also, SF5 building blocks will be cheaper and more readily available
thus enabling more research into novel pharmaceutical (and agricultural) candidates!
|
| |
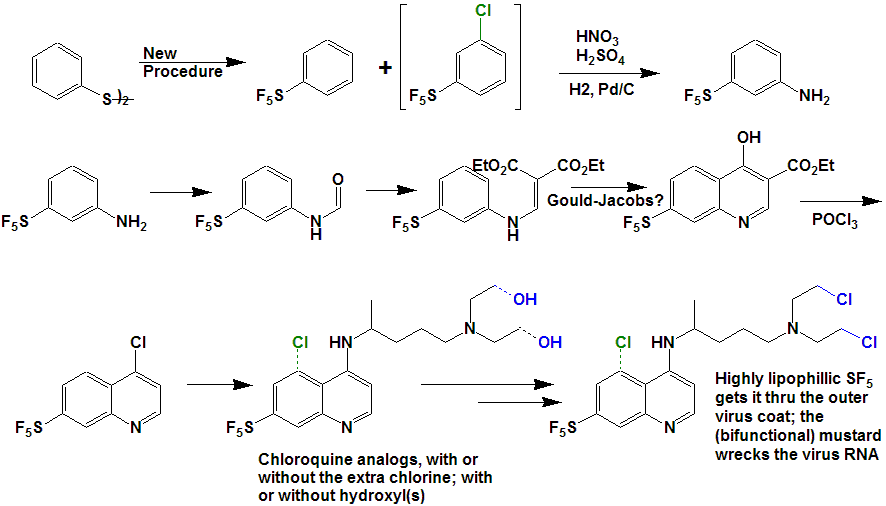
| Anti viral drugs may also benefit from SF5 substituents; here are some postulated analogs of Chloroquine: |
 |
| LLOYD GARRICK |
| |
| More links concerning bio-active compounds; CF3 vs. SF5 : |
|
|
|
| |
| |
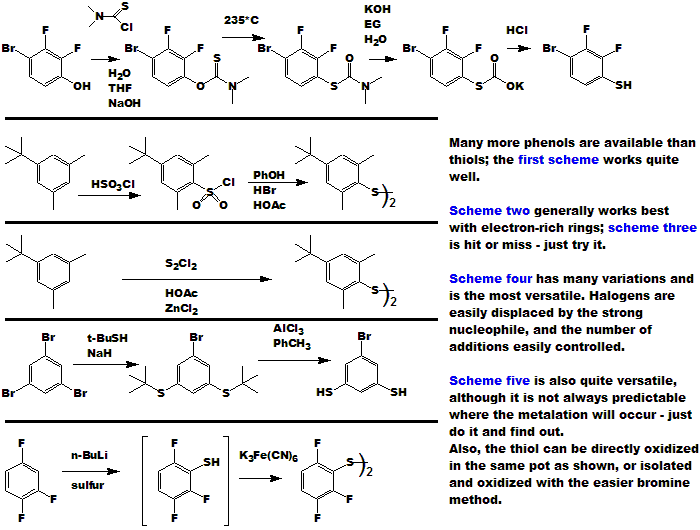
The New Preparation Methods
In order to contribute to and improve SF5 chemistry above, UBE has started to deliver a series of aromatic pentafluorosulfanyl
compounds prepared by new innovative processes including our KF/Cl2 method, which was developed by IM&T Research Inc.
|
| LLOYD GARRICK |
 |
| LLOYD GARRICK |
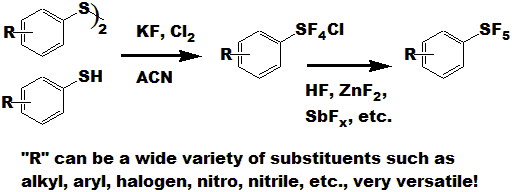
Our patented KF/Cl2 method is widely applicable to various aromatic disulfide compounds, which are direct
starting materials for the corresponding aromatic SF5 compounds. This has enabled us to introduce the SF5 group into various aromatic rings
via a two step process from the corresponding aryl-disulfide, as compared to the direct fluorination process
utilizing elemental fluorine, which is limited by the use of only nitro-aryl compounds as starting materials. |
With the KF/Cl2 process, aryl-disulfide is converted to the corresponding aryl tetrafluorosulfanyl- chloride. This process is equally
applicable to aromatic thiophenol compounds. The obtained Aryl-SF4Cl from the KF/Cl2 process can then be converted to the corresponding
aryl-pentafluoro-sulfanyl compound with zinc difluoride or anhydrous HF. Aryl-SF4Cl preparation proceeds with high yield around 80-90% at room temperature,
and the starting materials provided for this reaction, aryl-disulfide, KF and Cl2, are commodity materials, which can be obtained conveniently and at relatively low
prices for industrial scale production.
|
The conversion to Aryl-SF5 from the corresponding Aryl-SF4Cl,
proceeds with high yield (around
70-80%) with zinc difluoride at 100*C, and it also has been demonstrated
that this reaction proceeds with aHF in high yield (70-75%) below 20*C.
Certain other metal fluorides,
and mixtures thereof, have been found to work as well.
The following links will take you to the detailed descriptions of the new procedure(s):
The original paper detailing the processes can be accessed here.
This UBE patent details the procedure(s) for the poly-functional SF5 compounds. I must point out here that I did all of the
hands-on lab work and development, not most of it, all of it,
100%. Due to UBE policies and legal technicalities, my name is not
listed; only the author of the original concept is credited.
And the same deal with this one. [Yes, that sucks, but it is the real world]
In addition, I have some new research ideas which would not only improve even the existing new process significantly, but would also skirt the existing patents - all I need is a lab to develop the "new process"!
[And as I no longer work for UBE, I can thus work for you !]
Fetch the Acrobat PDF File of the original BeilStein article.
And don't try to save or download this web page!
Do it the EZ way ---
Just download the whole thing as a complete PDF file!
Incorporation of SF5 into Explosives and High Energy Compounds and Molecules
As a part of continuing research for energetic materials that combine high performance with low
vulnerability toward accidental detonation, the effect of introduction of the pentafluorosulfanyl (SF5)
group on the properties of explosive nitro compounds has been reported. This is based on the fact
that more energy is released due to the formation of HF in the detonation of SF5 explosives.
It is also well - established that substitution of H by F in hydrocarbons leads to a
significant increase in density. It implies that SF5 group would provide nitro
explosives with higher density or, in other words, improved performance, as explosive "power"
is proportional to the square of the compound density. To illustrate, PhSF5 has fully twice the
density of the analogous PhCH3 (toluene).
Based on these assumptions, some polynitro SF5 explosives have been designed
and their performance predicted which is in agreement with their actual
performance. The initial experiments of Sitzmann et al. support the hypothesis
that the SF5 group may provide explosives with improved properties: increased density,
increased insensitivity and increased energy coupled with better thermal stability – a
unique combination of properties. Some examples of dense, thermally stable,
impact insensitive polynitroaliphatic explosives with SF5 groups are:
SF5CH2CO2CH2C(NO2)2F density 1.86 gcm-3
SF5CF2CF2CF2SF5 density 2.04 gcm-3
(SF5)2NCF2CH2SF5 density 2.13 gcm-3
Corresponding work with aromatic compounds, although limited at this
time, has given similar results; this is a wide-open area for research
now, and the new production processes for aromatic SF5 compounds will be applicable.
References
- Bement , L.J. ( 1970 ) Application of
temperature resistant explosives to NASA
missions . Proc. Symp. on Thermally
Stable Explosives, Naval Ordnance
Laboratory, Whiteoak, Md, 1970 .
- Urbanski , T. ( 1984 ) Chemistry and
Technology of Explosives , vol. 4 , Pergamon
Press , Oxford, UK , p. 206 .
- Davenas , A. ( 2001 ) Solid Propellants for
Future Space Applications , European
Space Agency, Special Publication – 484
(SP - 484), pp. 105–110 .
LLOYD GARRICK
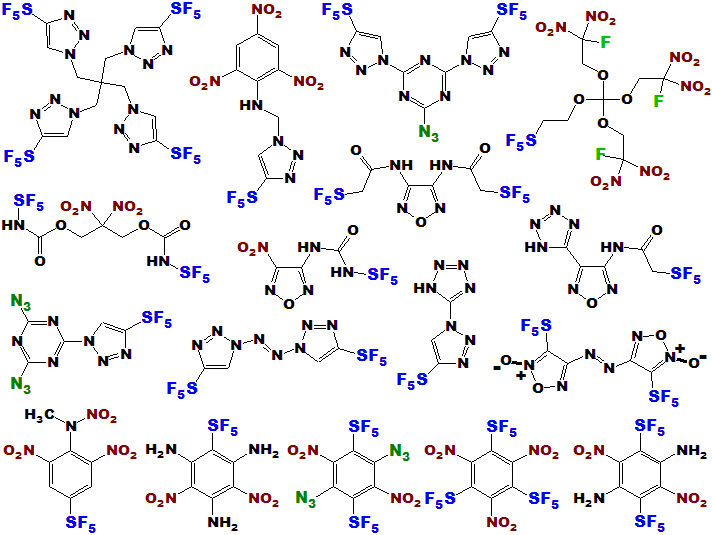
Recently, there has been increased interest regarding the incorporation of the SF5 group into energetic materials.
It is known that the inclusion of SF5 generally increases the thermal and chemical stability of organic molecules,
and in addition to this, it has been demonstrated that the presence of SF5 also will increase the density, and thus
the performance of the energetic material, as such performance is a function of the square of the density.
The possibility of a higher density, larger energy release, and better thermal and chemical stability without increasing
the sensitivity make the SF5 group attractive in the synthesis of high energy materials. (explosives).
The high fluorine content along with the presence of hydrogen leads to the formation of hydrogen fluoride (HF) upon
detonation, generating a large amount of energy. The S-F Bond Dissociation Energy (BDE) is 79 kcal/mol, while the
BDE of H-F is 136 kcal/mol. Much energy is also released in the formation of C-F bonds, as well as Al-F, if aluminum
(or other metal) is included in the formulation.
The formation of C-F, H-F or Al-F bonds, which have a higher bond dissociation energy (BDE) than the S-F bond in the
pentafluorosulfanyl group, allows the release of large amounts of energy upon detonation. This combined with the
possibility of higher density, higher thermal and chemical stability and low sensitivity makes the pentafluorosulfanyl
group very attractive for the synthesis of high performance energetic materials. (explosives).
A large number of SF5-containing energetic materials have been synthesized where the predicted performance is close
to those for HMX, RDX and TNT, but with the benefit of lesser or no impact sensitivity.
Most of these are built up from triazoles, furazans, and other high-nitrogen/oxygen heterocycles, which, if the corresponding
thiol is available, would be easily accessible by these new methods, thus accelerating research efforts.
LLOYD GARRICK
 LLOYD GARRICK
LLOYD GARRICK
References
-
Sheppard, W. A. J. Am. Chem. Soc. 1962, 3064.
- Ye, C.; Gard, G. L.; Winter, R. W.; Syvret, R. G.; Twamley, B.; Shreeve, J. n. M.
Org. Lett. 2007, 9, 3841.
- Garg, S.; Shreeve, J. M. J. Mater. Chem. 2011, 21, 4787.
- Agrawal, J. P. Prog. Energy Combust. Sci. 1998, 24, 1.
- Sitzmann, M. E.; Gilligan, W. H.; Ornellas, D. L.; Thrasher, J. S. J. Energetic Mat.
1990, 8, 352.
- Sitzmann, M. E. J. Fluorine Chem. 1991, 195.
- Gao, H.; Ye, C.; Winter, R. W.; Gard, G. L.; Sitzmann, M. E.; Shreeve, J. M. Eur. J.
Inorg. Chem. 2006, 3221.
- Ornellas, D. L. Propellants, Explosives, Pyrotechnics 1989, 14, 122.
Chemistry; SF5 in explosive compounds:
- Pentafluorosulfanyl polynitroaliphatic urea, monocarbamate, and dicarbamate explosive compounds
- Polynitroaliphatic explosives containing the pentafluorosulfanyl (SF5) group: The selection and study of a model compound
- Recent Trends in New Energetic Materials
- 1,3,4-Oxadiazoles containing the pentafluorothio (SF5) Group
- Pentafluorosulfanylnitramide Salts
- Pentafluorosulfanyl Polynitroaliphatic Urea Explosive Compounds
- Pentafluorosulfanyl Monocarbamate and Dicarbamate Explosive Compounds
- Pentafluorosulfanyl Carbamate Explosives
- High Energy Pentafluorosulfanyl Polynitroaliphatic Urea Monocarbamate and Dicarbamate Compounds
- Pentafluorosulfanyl-substituted-poly-123-triazole-compounds

Go Back to Main Page
Following are some ideas for high energy compounds:
LLOYD GARRICK
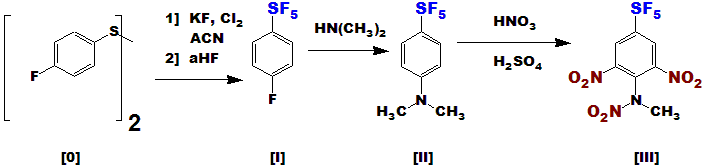
[III] is the SF5 analog of TETRYL, which has a NO2 instead. TETRYL is a very powerful secondary explosive,
once in widespread use, but no longer as it is too unstable and sensitive.
Since SF5 is known to stabilize these types of compounds, [III] is reasonably expected to be a very powerful
tertiary explosive, stable enough for use; it's main advantage here is the ease of synthesis !!!
[I] is made from the readily available [0] by the new process(es), then reacted with dimethylamine to produce [II].
Substitution of fluorines on these SF5 compounds by nucleophiles generally procedes well - I have done countless such reactions.
Then, the activating and directing effects of the amine and SF5 enable nitration to procede under relatively mild conditions
(similar to the TETRYL reaction from dimethylaniline) to yield the product [III].
Next Generation Explosives that are EASY to Synthesize!
LLOYD GARRICK
LLOYD GARRICK
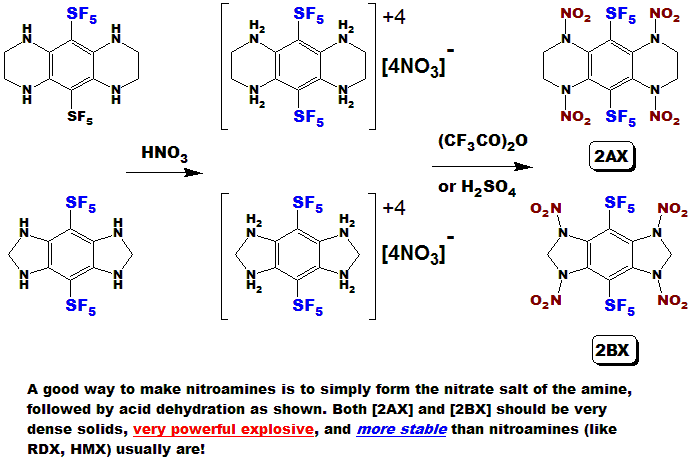
LLOYD GARRICK
I have proceeded to the bifunctional compound [V] in good yield, it is a clear oil, pleasant odor.
The dinitration should be straightforward, I just haven't done it yet - I need a lab!
Compound [VII], analogous to the known and currently used TATB,
would be even more stable, and very likely more powerful !
And lookie how EASY it is to make !
Next Gen Explosives Easy to Make!
LLOYD GARRICK
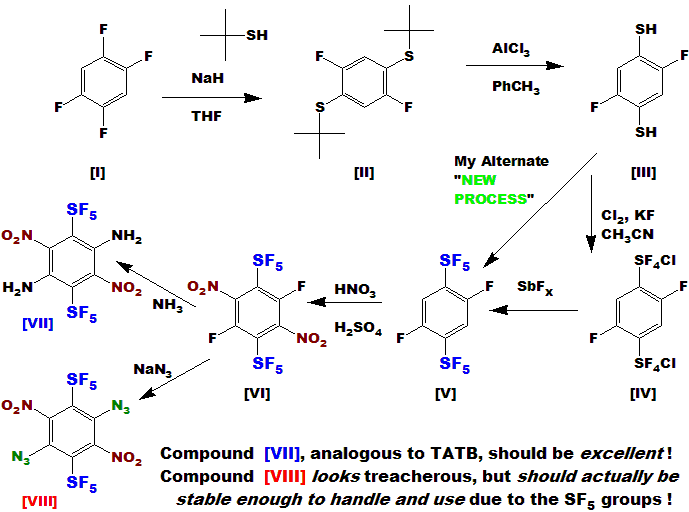
LLOYD GARRICK
Compound [I] is commercially available; nitration to [II] should be easy, facilitated by the fluorines.
Formation of [III] will be easy; I have done many such reactions. Deprotection reaction to [IV] would
normally use catalytic (5 mol %) AlCl3, however the nitro groups may coordinate also, thus
stoichiometric quantity may be needed; alternatively, this deprotection can also be done with TFA or HF.
The new processes can then form the SF5 compound from the thiol.
Although nitration can easily be done ortho to SF5, (I have done it), the converse is sometimes difficult.
Steric effects can interfere with the formation of the intermediate SF4Cl. Don't know here - I need a lab to find out!
Compound [VI] should be very dense due to the SF5 groups and the symmetry; since explosive power is proportional
to the square of the density, this compound should be an extremely powerful explosive - perhaps the best yet (non nuclear).
It also has an excess of oxidant, thus it could be formulated with
aluminum, titanium, magnesium, etc., further increasing the power.
I have several alternate approaches to this molecule. If I only had a lab .............
LLOYD GARRICK
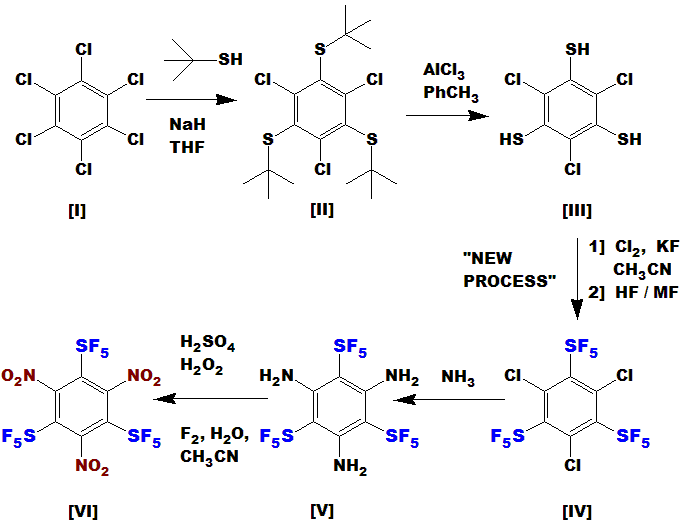
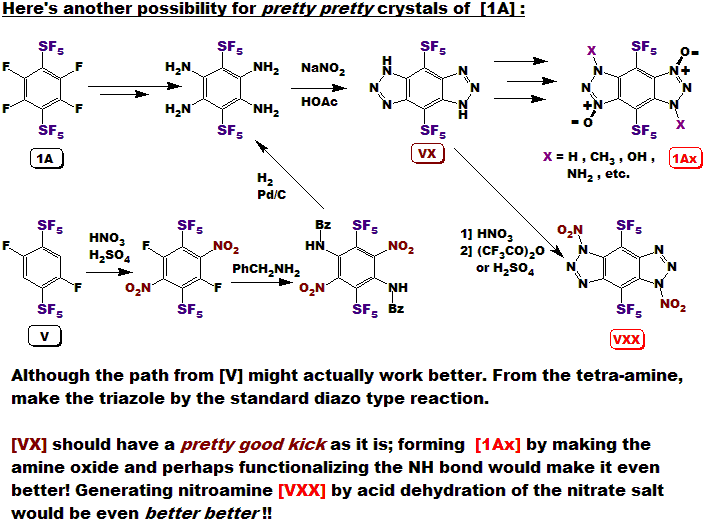
Another way to do it:
Hexachlorobenzene is commercially available and cheap; hexafluorobenzene would actually work better, but it is more expensive.
Hexabromobenzene would prevent the 3rd step (formation of SF5) due to steric hindrance.
Proceeding to compound [III] would be uneventful – this reaction works quite well and I have done it countless times.
The new UBE/Umemoto process (or my new process) would then make the trichloro tri(pentafluorosulfanyl) compound [IV].
Reaction with NH3, possibly aided by NaH, should form [V]. This reaction works well with the analogous nitro compound;
as SF5 is similar to NO2 in electronegativity, I expect it also to work here.
Oxidation of amines to nitro is effected by the “persulfuric acid” mix; this drastic reaction condition should not affect the rock-stable SF5.
Alternatively, the much milder fluorine/acetonitrile/water oxidizer is
known to effect this conversion well, I just haven’t tried it yet – I need a lab!
LLOYD GARRICK
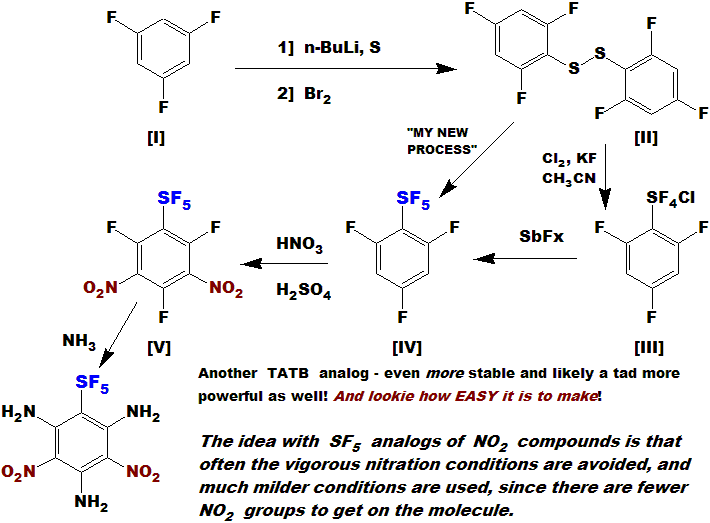
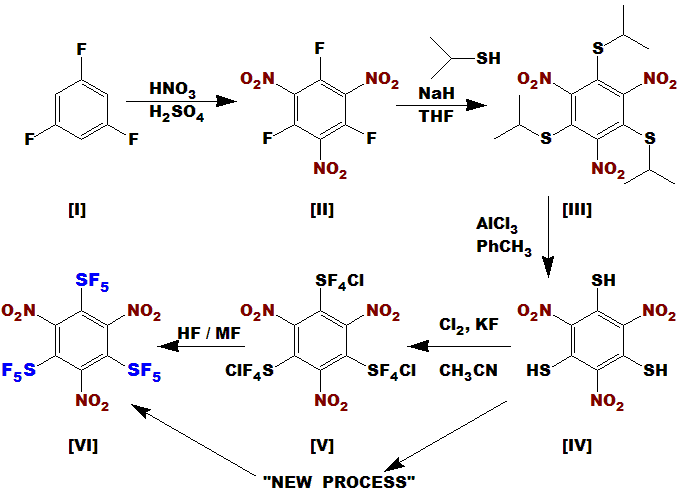
Compound [I] is commercially available also; I have made multi-dozen gram lots of [IV]. Like most of these compounds,
it is a clear fragrant oil. The fluorines are activating and direct ortho, and there are three for additive effect.
SF5 is deactivating and directs meta; all effects point to the open positions, thus this dinitration should
also be straightforward; again - I just need a lab!
I expect [V] to be a dense explosive solid, very stable also, perhaps difficult to detonate even.
The fluorines are very labile, and could easily be
substituted with appropriate nucleophiles giving even better compounds. Some possibles are phenyl or triazine with ring nitros
or azides, using an amine, hydrazine or diazo bridge. Heck - simple amine or hydrazine should work nice! Even azide!
Compound [VI] might start looking real treacherous with these substituents, but remember - the SF5 is stabilizing it!
.
LLOYD GARRICK
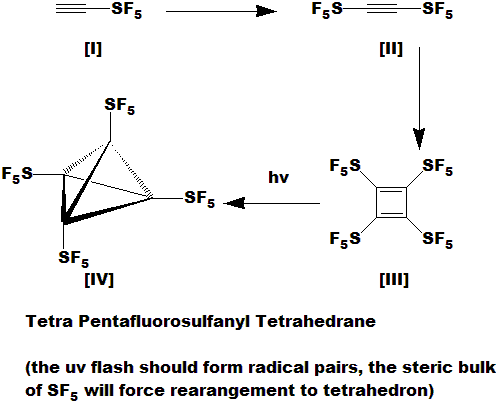
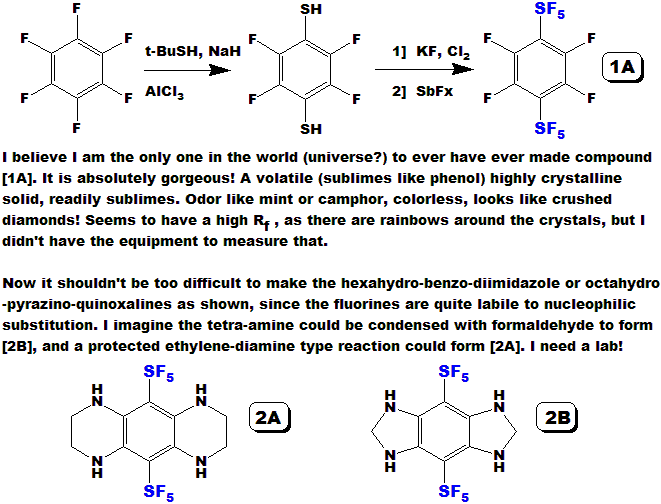
Tetra Pentafluorosulfanyl Tetrahedrane
[I] and [II] are known compounds; I haven't worked with them yet. They are made from ClSF5. Et3B, LiOH;
addition/elimination reactions on unsaturated compounds. Many variations, and much room for improvement yet.
I don't know how to get from [II] to [III]; perhaps the right cobalt or palladium complex should do it; it looks like a simple [2 + 2].
[III] is of course anti-aromatic, thus unstable and prone to polymerization and decomposition, however,
the electron pull of the SF5 groups might stabilize it somewhat; if I can get it into the photolysis apparatus
quick enough, a blast of 254 should create [IV].
I expect [IV] to be a dense, highly explosive solid; perhaps too unstable for practical uses; it may not be possible to exist at all.
Currently, only the tetra-t-butyl-tetrahedrane has been made. Despite considerable effort, the tetra-nitro and parent compounds
have eluded synthesis. The electron pull of the SF5 groups would further weaken the already highly strained cage and
might make this compound impossible. Don't know - I need a lab!
LLOYD GARRICK
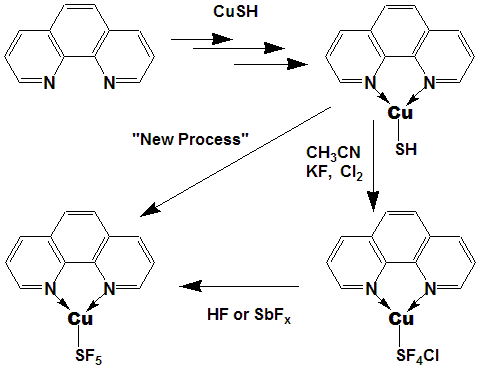
It's a longshot but I'd like to try it anyway!
The crazier it looks the more fun it is to play with.
We have trifluoromethyl transfer agents; how about a
pentafluorosulfanyl transfer reagent!?
The two functions share many of the same characteristics,
and copper likes bonding to sulfur - it just might work!
[although I have never used the new processes on metal-sulfur
bonded compounds - no idea what to expect], but isn't that
what research is all about!?
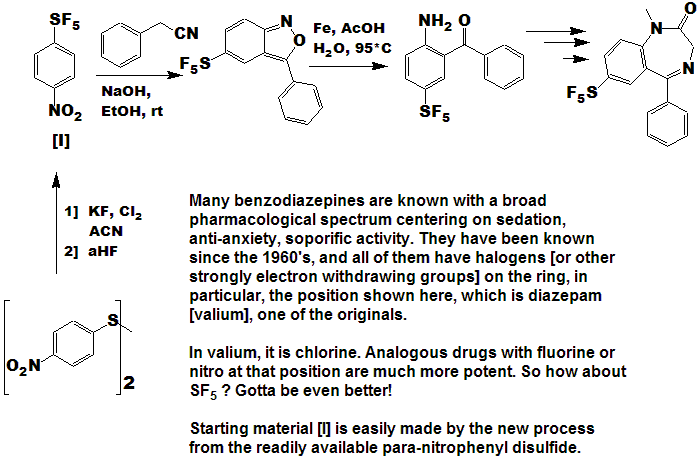
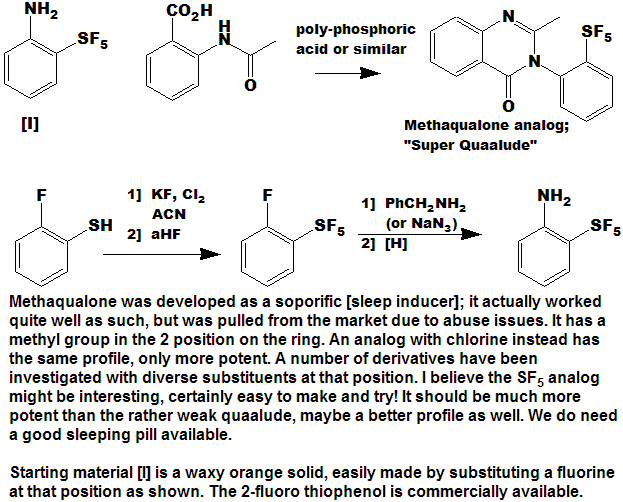
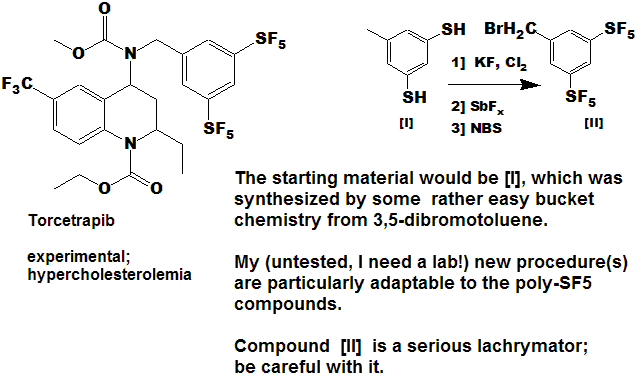
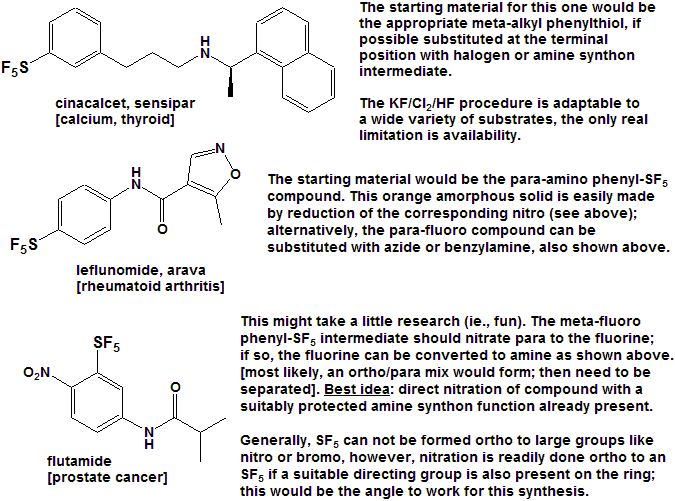
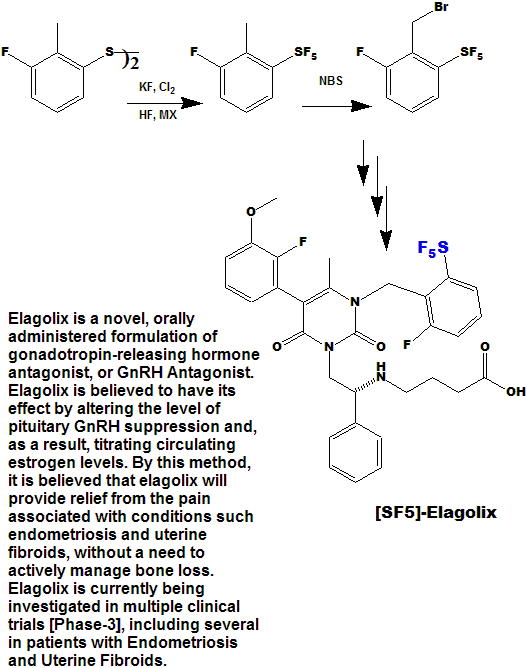
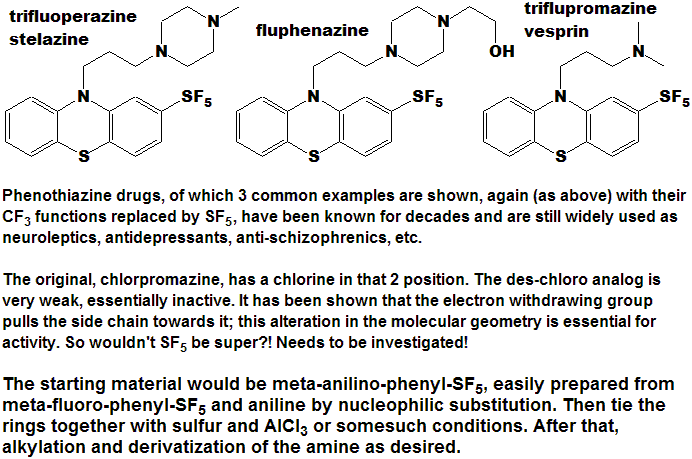
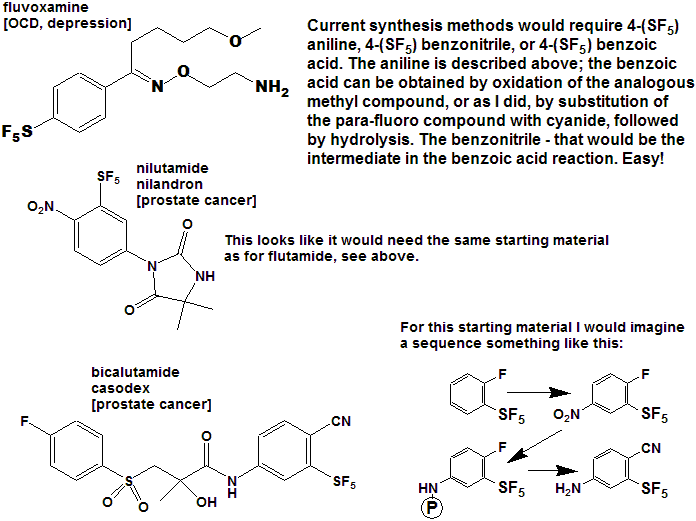
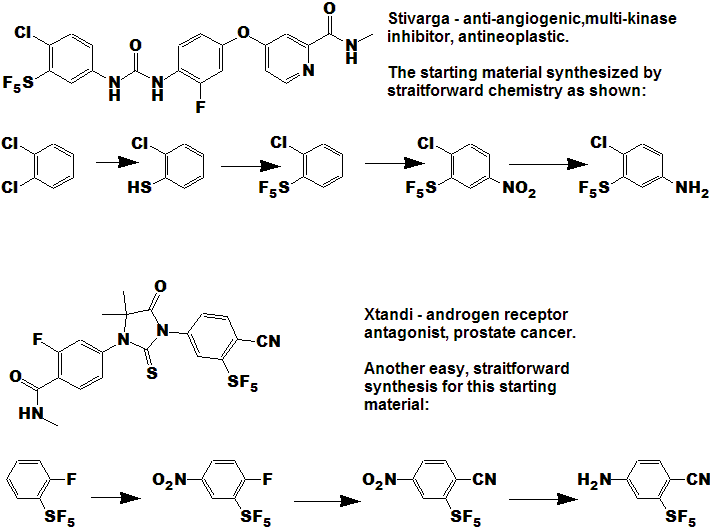
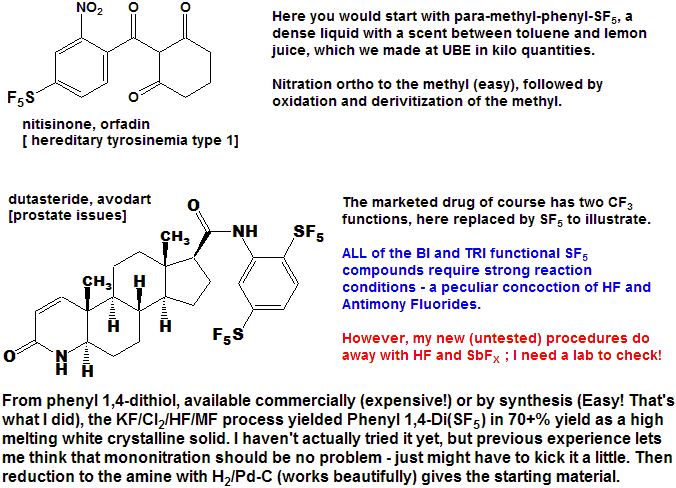
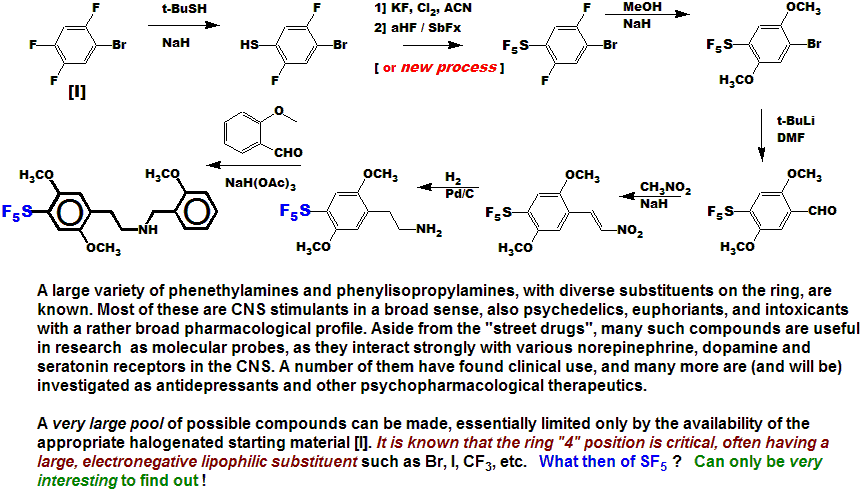
Quite a number of currently marketed drugs [and more will be coming!) contain the CF3 function; some examples are depicted below, with the CF3 replaced by SF5 to demonstrate hypothetical concepts.
Now here is an idea for bold, daring entrepreneurial spirited individuals and/or companies :
- Take any currently marketed drug [or agricultural compound] containing CF3, make the SF5 analog, and submit it as a new compound.
- It is a better than even bet that it will be a significantly better compound, enough so that the FDA will accept it as such, and not just another "copycat".
- Since all of the research, time, expense, and manpower has already been done on the "rest" of the molecule, you will be spared that !
- And it is unlikely that the original patent(s) would cover
the carbon-sulfur bond at that position, (but you will need to verify
that to avoid legal issues).
- You get a fast-track short-cut one-up on the competition in the cut-throat competitive arena of drug development !
- I could do this myself, but as I said above - I need a lab !!!
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
LLOYD GARRICK
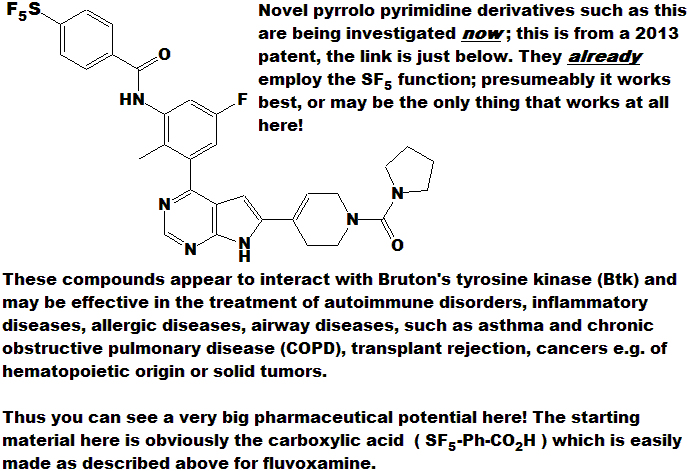
See the full WO 2013008095 A1 patent HERE.
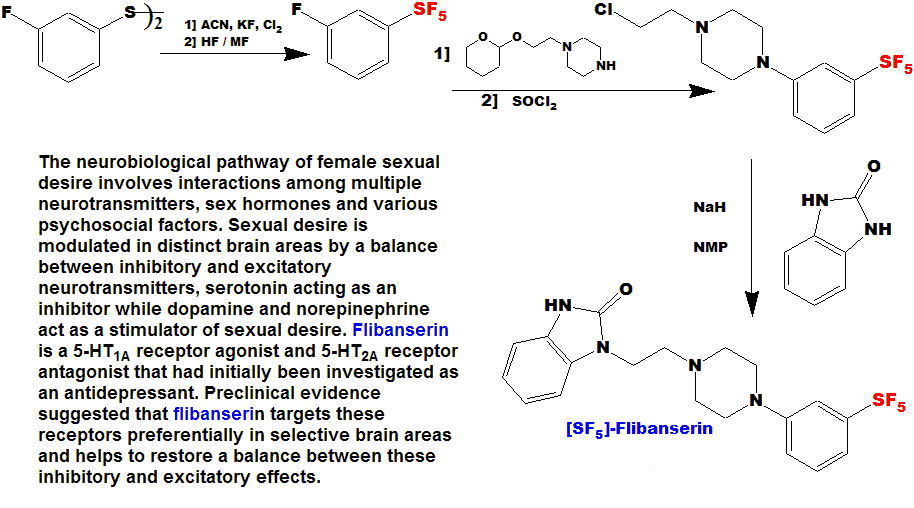
[ SF5 ] - Flibanserin
LLOYD GARRICK
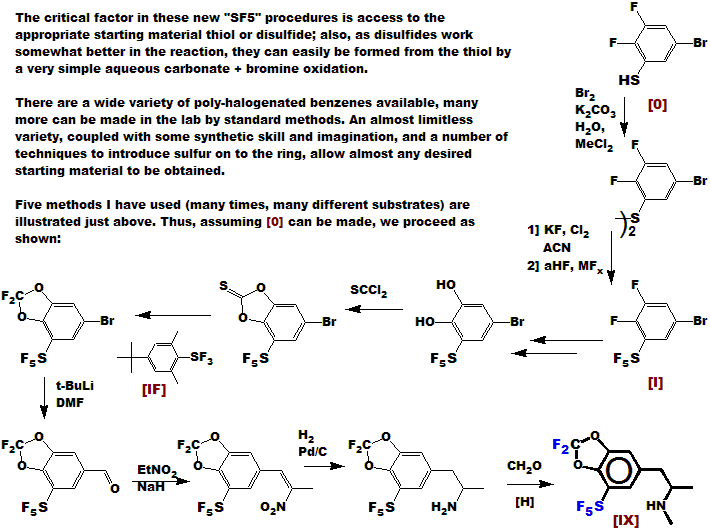
Compound [IX] has got to have a very interesting pharmacological (CNS) profile!
The interesting, but scarcely known and used difluoro-methylene-dioxy function is
conveniently made using the new FLUOLEAD deoxofluorinating reagent [IF],
which we developed at UBE concurrently with the new SF5 process.
WebSite by Lloyd Garrick.
Created with NotePad.
Updated 01/16/2019.

Go Back to Main Page
Aromatic SF5 Perfluorosulfanate Pentafluorosulfanate Sulfanyl
Pentafluoride Sulfurpentafluoride Perfluorosulfanyl
Pentafluorosulfanyl SF5 Lloyd Garrick explosives explosive
propellant rocket fuel high energy FLUOLEAD agricultural
pharmaceutical medicinal drug drugs
|
| |
| |
|